Abstract
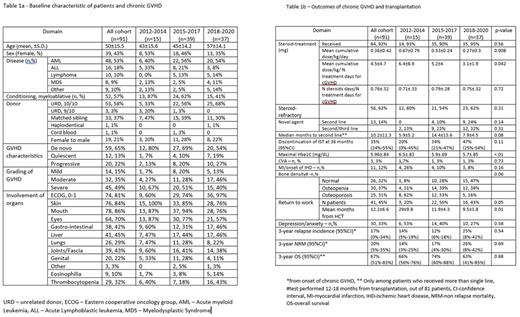
Background - Chronic graft vs host disease (cGVHD), explicitly steroid-refractory disease, is associated with substantial morbidity and mortality in patients undergoing allogeneic hematopoietic cell transplantation (HCT). Since 2018, access to novel immunosuppressive therapies (IST) for steroid-refractory GVHD have become available via clinical studies, early-access programs and private insurances. We aimed to analyze changes in treatment pattern and thus outcomes during the last decade in this population. Methods - We included all patients above the age of 18 years who developed cGVHD after first allogeneic transplantation performed between 2012 and 2020 in the Tel Aviv Sourasky Medical Center. We divided the patient cohort into 3 treatment periods according to clinical trials available/FDA approved drugs - 2012-2014 (group 1), 2015-2017 (group 2), and 2018-2020 (group 3). The NIH 2004 criteria was used to define chronic GVHD. All patients were managed by the same medical team with a well-established long-term survival program. Data was extracted on July, 2022. Results - between 2012 and 2020, 301 patients underwent HCT, of them 222 survived more than 3 months and 91 (41%) developed chronic GVHD and fulfilled the inclusion criteria. Median follow-up was 51 (range, 13-97) months. There was no statistically significant differences in patient characteristics between the 3 groups, except of older age and greater employment of reduced intensity conditioning in patients included in group 3, Table 1a. All patients, except 1, received GCSF-moblized peripheral blood stem cells and all patients receiving matched-unrelated donor allografts, were given anti-thymocyte globulin. 92% of patients were managed with steroids as first line treatment for cGVHD. Patients in the second and third group, compared to the first, received lower mean cumulative dose of steroids/kg/day (p=.008) and fewer mean number of treatment days for cGVHD (p=.042). However, there was no difference between the groups in the proportion of number of steroids treatment days/total days of treatment of cGVHD (p=0.72), Table 1b. The median IST-free survival was 79 (95% CI 54-94) months, with a higher percentage of patients in group 3 able to discontinue IST at 3 years from GVHD onset, though this did not reach statistical significance (p=0.1). Regarding long-term complications, patients in cohort 2/3 had better diabetes control (p<0.001), a trend for lower cardiovascular complications, and a higher bone density (p=0.06). A significantly higher proportion of patients returned to work in cohort 2 and 3 (56% and 43%, respectively) compared to cohort 1 (20%) in a shorter period of time from HCT (p=0.05 and p=0.01). There was no difference in the incidence of depression/anxiety. There was no difference in 3-year relapse incidence (p=0.54), non-relapse mortality (p=0.69) and overall survival (0.68). Cox regression analysis identified lower platelets at the onset of cGVHD and the employment of reduced intensity regimen, as being associated with shorter survival (P=.005, HR-1.1 and p=.049, HR-3.5, respectively), while patient's group, sex, age and eosinophil count ,did not impact survival. Conclusions - this real-world data clearly show that incorporation of FDA-approved novel agents for the management of cGVHD is associated with reduced overall steroid consumption and a better control of metabolic long-term complications. While these effects significantly improve some aspects of quality of life, these modifications in therapy are yet to be translated to survival advantage.
Disclosures
Shasha:GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astelas: Speakers Bureau; Biotest: Honoraria. Verssano:GSK: Membership on an entity's Board of Directors or advisory committees. Avivi:Kite, a Gilead Company: Speakers Bureau; Novartis: Speakers Bureau. Ram:Takeda: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal